One of my great frustrations is to see start-ups fall at the final hurdle. Too many early-stage companies with promising innovations spend their energy – and funds – answering crucial questions about the safety and effectiveness of their products, only to find that they have no answers to the additional questions asked by payers.
HTA bodies and procurement officers need to know the real-world impact of a new technology. How will it influence patient outcomes and experience? How will it change workflows and how clinicians spend their time? How will it shape the efficiency of the health system?
If a researcher or entrepreneur cannot answer these questions, nobody cares how cool their gadget is. This is a tragedy. Great ideas wither and die; innovators flee for the US or Asia. From a European perspective, I see it as a waste of intellectual energy and potential innovation that could add much-needed value.
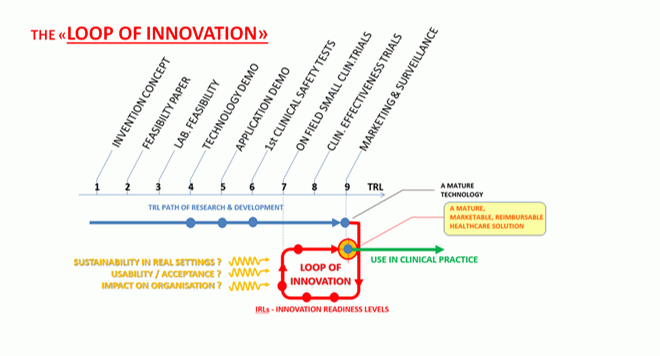
I don’t blame the start-ups. They are working within an outdated framework that tells them their technologies are ‘mature’ when they reach what’s known as Technology Readiness Level 9 (TRL9) – a commonly used conceptualization of the path from idea to market. But, in reality, the road to TRL 9 ends before the widespread adoption of a medical technology. TRL 9 is not the destination; it’s just the end of the beginning.
Introducing the iLoop
It is time for a rethink. Looking at the bottlenecks facing late-stage research groups and early-stage companies, I believe the solution is to marry the development of evidence supporting CE marking with the generation of real-world evidence demanded by payers.
That’s where the Innovation Loop (iLoop) comes in. When companies begin testing their prototypes, they should also measure the impact of their technology on the healthcare organisation; its usability and acceptance; and its sustainability in a real-world setting.
To put this into practice, we buy prototypes work with companies to guide them through the iLoop. Along the way, we seek improvements and refinements to ensure the technology can be integrated and scaled up.
We’ve already done this with rehabilitation robots in 30 rehab centres, generating data on 60,000 treatments of 3,500 patients. Not only is an ‘innovation trial’ of this kind an opportunity to see how the device works, the robots can collet data on how they are used. By comparing with data from before the robots were introduced, we can see whether patient outcomes improved and whether therapists’ time was used more efficiently.
Our team has also created dashboards that visualize the data showing whether the device offers value. Presenting this in the form of a heatmap offers decision-makers instant insights on whether a technology represents value for their service.
Ultimately, we see this as a catalyst for value-based procurement which promises to improve outcomes for patients while optimizing health system efficiency. For start-ups too, this is way to overcome a major barrier to innovation adoption. The iLoop could be a win-win for medtech innovation in Europe.
Check out other blogs on Research & Innovation here.








