58 results

Posted on 05.11.2013
Europe’s most vibrant startups are in medtech
Does Europe have a proper start-up culture? Are we doing enough for young professionals? According to Neelie Kroes, Vice-President of the European Commission, in her article for the World Economic Forum, we could do more to make Europe fertile ground for start-ups, and engage the youngest generation entering the workforce. As a young professional in Brussels well-acquainted with millennial woes, I was eminently interested in the views of an established and influential official at the European Union.

Who’s afraid of the big bad data?
Two years ago in our annual report on the medtech sector, Pulse of the industry, we warned that the sector was facing a perfect storm, caused by a general shift toward value-based care, growing regulatory pressure in the US and limited resources as a result of a global downturn. Those events came to pass, with the added complication of tougher new regulatory issues in Europe. It was time, we felt, in this year’s report to see how the sector is weathering the storm.

Posted on 12.09.2013
Pacing the implementation of the new IVD regulations
Getting the timing right for the implementation of changes is essential to ensuring that the new regulations result in a better system for IVDs rather than a bureaucratic quagmire. The sweeping changes being made to risk classification will be complex and time-consuming. The lessons learned from similar overhauls in other markets show us that a period of transition is essential to optimise the implementation of changes for the new European IVD legal framework.

Posted on 04.09.2013
Innovating the way we innovate
Value creation capabilities based solely on R&D investment are not generating as much growth as they used to. According to PwC’s “Operating performance in the Medtech industry: Trends and imperatives” report, which studied the performance of 56 global medtech companies, the impact of R&D on revenue growth declined at an average annual rate of 10% and the return on invested capital declined at a rate of 2% between 2005 and 2011. The impact on growth is evident by revenue growth rates declining at a rate of approximately 12% per year.

Posted on 20.08.2013
My first twelve months at EDMA and Eucomed
While enjoying my holidays I was reminiscing a bit about my time in Brussels and thought I‘d share my reflections with you. A little over a year ago, on 16 July 2012 to be precise, I joined EDMA and Eucomed. As such, this “fait divers” is not that important, but since then a lot has changed that will impact our industries and associations.

Posted on 02.05.2013
Eurasian Economic Integration – market opportunity or regulatory challenge for the MedTech industry?
Until recently economic and regional integration in the post-Soviet countries to a large extent was only declarative. However, the initiative of going beyond the Customs Union (CU) to create Common Economic Space (CES) between Russia, Belarus and Kazakhstan draws attention to potential market opportunities and at the same time regulatory challenges for the MedTech industry.

Getting regulation right for in vitro diagnostics and medical devices
It’s been a busy two weeks at the Parliament once again, with the release of both the draft report for the revision of the Medical Devices Directives (MDD) and that for the In Vitro Diagnostics Directives (IVDD) revision. Last Monday, EU Parliament Rapporteur Dagmar Roth-Behrendt published her draft report on the revision of the MDD. Although not surprised, we of course regret to see proposals such as a shift to centralised pre-market authorisation. This US-like system will not bring about the necessary additional patient safety and actually risks hindering patients in their access to lifesaving devices because of unnecessary delays in these devices becoming available to them.

Medical devices and in vitro diagnostics proposals: Information is Power
World Health Day approaching us on April 7th is meant to raise awareness on the endless host of hurdles that undermines our health and therefore, our quality of life. Public health is about making sure that the external factors that determine our health are governed by policies that have people’s well-being at the very heart of their mandate. EPHA, Europe’s leading NGO advocating for better health, is committed to that very principle.
This year marks EPHA’s 20th anniversary in advocating EU and European policy-makers on public health. Bringing today’s leaders to focus on people’s health is not an easy task. The medical devices and in vitro diagnostics files appropriately represent the juggling effort that advocating for public health usually turns out to be.
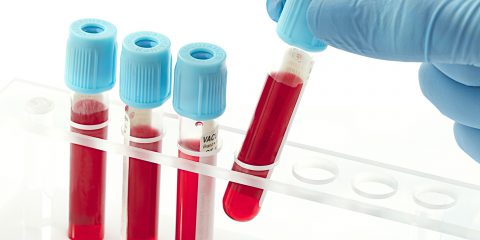
Understanding in Vitro Diagnostics & Why They Need a Separate Regulatory Framework
Many times I’ve found myself in situations where I had to explain that indeed in vitro diagnostics (IVDs) are to all extent medical devices but that, in practice, the subject we happened to be discussing would not entirely (or at all) be applicable to IVDs.
It was always a case of: “…yes, yes.. BUT”…
If there is one message you take away from this post, let it be… IVDs have very different risk sets associated to them: no direct contact with patient, value of the medical data they deliver and that they provide no treatment.











